Hospitals brace for IV fluid shortages after Hurricane Helene closes North Carolina manufacturing plant
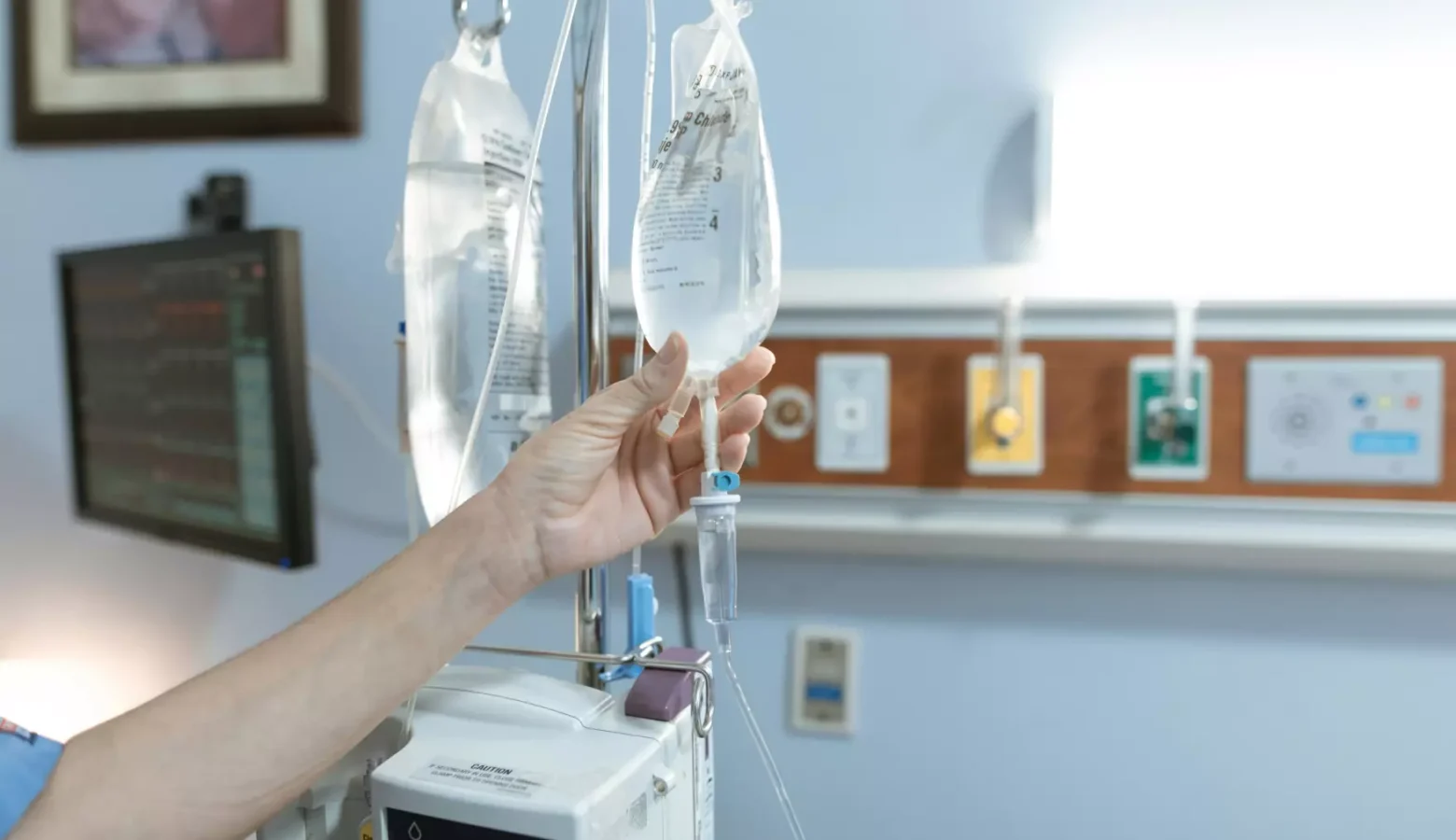
Hospitals across the U.S. are bracing for a potential shortage of intravenous fluids, also known as IV fluids, after Hurricane Helene brought the country’s biggest manufacturing plant –– responsible for nearly 60% of IV fluid supply –– to a halt.
It’s the latest example of a natural disaster’s impact extending well beyond its immediate human toll and structural damage.
“This is definitely the number one issue for hospitals across the country right now,” said Soumi Saha, senior vice president of government affairs at Premier, a group purchasing organization for medical supplies serving thousands of facilities across all 50 states.
“The biggest unknown right now is how long the shortage will last. Is this something that is two weeks and can be mediated quickly, or is this something that’s going to be impacting the healthcare supply chain for years to come?”
Baxter International, the largest maker of IV fluids in the U.S., said its manufacturing plant in North Carolina, which produces 1.5 million IV fluid bags per day, was damaged by the hurricane. The heavy rain and storm surge triggered a levee breach, which led to water permeating the site. The bridges leading to the site have also been damaged.
Efforts to conserve
IV fluids are a cornerstone of patient care. The fluids, which are infused with salt, carbohydrates, and electrolytes, are injected through a tube into the patient’s vein to address a plethora of medical conditions. For example, they are used to treat or prevent dehydration, deliver medications, hydrate patients before and during surgeries and remove waste from the blood for kidney dialysis patients.
It’s why hospitals are on alert.
The American Hospital Association says it is “in frequent discussions with officials from Baxter, the Department of Health and Human Services (HHS), the Food and Drug Administration (FDA), and other federal agencies about the supply disruptions and what steps can be taken to assist hospitals and health systems as they care for patients”.
Some hospitals have started implementing conservation plans.
Dr. Ryan Stone, chief medical officer at Indiana-based Schneck Medical Center in Seymour, said his hospital is fully reliant on Baxter for its supply of IV fluids. He said the hospital was notified that all orders were being put on allocation and that they would get just 40% of their previous orders.
Stone said the hospital is being more conservative with its IV fluid usage –– switching patients to oral hydration as soon as possible and using different IV fluid bag sizes to reduce waste. Stone added that his hospital hasn’t seen the shortage impact patient care yet.
“If we want to try to conserve, now is the time to do that before we get into a scarcity standpoint,” he added.
Drug shortages are not new
The FDA said it may look at temporarily allowing imported IV fluids to mitigate shortages, according to NPR. But there are global shortages of IV fluid, and a lot of the manufacturing in the European Union is already at maximum capacity to offset some of these shortages, including in places outside of Europe, like Australia, said Saha of Premier.
“We can’t necessarily turn to our traditional partners to increase capacity, because they are already maxed out,” she said.
And unlike some other health care products, there is no strategic national stockpile of IV fluids, said Kyle MacKinnon, Premier’s senior director of operational excellence.
As of Oct. 7, the FDA hadn’t declared an official shortage of IV fluids in the U.S., a step that would lift some federal law restrictions and open up alternate pathways for producing the products.
There are some facilities across the U.S. that may have access to sterile water and systems in place to ramp up production. But without the FDA adding IV fluids to its official drug shortage list, these facilities won’t have the authority to begin compounding the products.
This is the latest example of the devastation natural disasters can cause to the U.S. health care infrastructure, and a reminder of how vulnerable the supply chain is.
In 2017, hospitals experienced a shortage of IV fluids after Hurricane Maria damaged manufacturing plants in Puerto Rico.
Beyond IV fluids, drug shortages are not new in the U.S.
But until recently, the federal government wasn’t really tracking drug stockpiles held by manufacturers, hospitals, or the Strategic National Stockpile. New pieces of legislation could help change that –– giving a better picture of critical medical supplies and pharmaceuticals on U.S. soil.
Health experts say the change could help the industry better avoid shortages.
“That’s a bit of an unknown right now,” Saha said. “We don’t really know what volume of product is in distribution channels and other supplier channels, what is sitting on shelves, and so we’re flying blind. And that was a lesson that we learned during the pandemic. We didn’t know how many ventilators were on U.S. soil. We didn’t know where they were.”
Impact on patients
Generally, most patients are likely not going to realize there is a shortage as hospitals rush to implement their conservation plans to ensure that they don’t reach a point of scarcity and that acutely ill patients continue to receive the IV fluids they need.
Patients with less acute illness, however, such as those who show up in the emergency rooms with nausea and vomiting, may be given oral hydration like Gatorade instead of IV fluids. Some hospitals in Minnesota are reportedly canceling elective surgeries too.
Baxter says they are working around the clock to get the factory operational again. There are currently 500 people on site, and this number is expected to double in the week ahead, the company says.
The FDA also said it’s working with Baxter and would expedite its review processes when the plant is cleaned and restored.
“FDA is also working with Baxter’s additional facilities to increase supply and reduce the risk of new shortages until Baxter can resume manufacturing the impacted products. FDA will also be working with alternative suppliers, as needed, to manage gaps/shortages in supply of critical products,” the agency said on its website.
Side Effects Public Media is a health reporting collaboration based at WFYI in Indianapolis. We partner with NPR stations across the Midwest and surrounding areas — including KBIA and KCUR in Missouri, Iowa Public Radio, Ideastream in Ohio and WFPL in Kentucky.


